Hint: The total no. of molecules will remain the same.
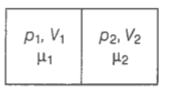
Consider the diagram,
Given,
Step 1: Find the relation between the pressure, volume and the total energy of the gas.
For chamber 1,
For chamber 2,
When the partition is removed the gasses get mixed without any loss of energy. The mixture now attains a common equilibrium pressure and the total volume of the system is the sum of the volume of individual chambers .
So,
From the kinetic theory of gases,
For 1 mole, [E=translational kinetic energy]
For moles,
For moles,
The total energy is,
Step 2: Find the final pressure of the chamber.
From the above relation,