Photoelectric effect When radiation with certain minimum frequency strike the surface of a metal, the electrons are ejected from the surface of the metal. This phenomenon is called photoelectric effect. The electrons emitted are called photoelectrons.
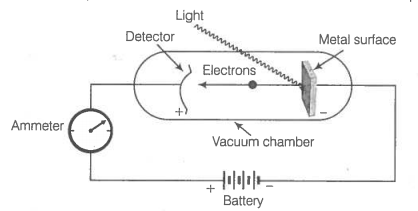
Equipment for studying the photoelectric effect. Light of a particular frequency strikes a clean metal surface inside a vacuum chamber.
Electrons are ejected from the metal and are counted by a detector that measures their kinetic energy.
The result observed in this experiment were
(i) The electrons are ejected from the metal surface as soon as the beam of light strikes the surface, i.e., there is no time lag between the striking of light beam and the ejection of electrons from the metal surface.
(ii) The number of electrons ejected is proportional to the intensity or brightness of light.
(iii) For each metal, there is a characteristic minimum frequency, (also known as threshold frequency) below which photoelectric effect is not observed. At a frequency , the ejected electrons come out with certain kinetic energy. The kinetic energies of these electrons increases with the increase of frequency of the light used.
The above observation cannot be explained by the electromagnetic wave theory. According to this theory, since radiations were continuous, therefore, it should be possible to accumulate energy on the surface of the metal, irrespective of its frequency and thus, radiations of all frequencies should be able to eject electrons.
Similarly, according to this theory, the energy of the electrons ejected should depend upon the intensity of the incident radiation.
Particle Nature of Electromagnetic Radiation
To explain the phenomena of 'black body radiation' and 'photoelectric effect', Max Planck in 1900, put forward a theory known after his name as Planck's quantum theory. This theory was further extended by Einstein in 1905.
The important points of this theory are as follows :
(i) The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy. Each such packet of energy is called a 'quantum'. In case of light, the quantum of energy is called a 'photon'.
(ii) The energy of each quantum is directly proportional to the frequency of the radiation, i.e.,
where, h is a proportionality constant, called Planck's constant. Its value is approximately equal to
(ii) The total amount of energy emitted or absorbed by a body will be some whole number quanta.
Hence, (where, n is any integer)