Among the following, the correct order of energies of molecular orbitals of N2 is:
1.
2.
3.
4.
Hint: Energy of σ 2 p z molecular orbital is more than π * 2 p x ≈ π * 2 p y molecular orbital .
Explanation:
Step 1: The MOT diagram for is as follows:
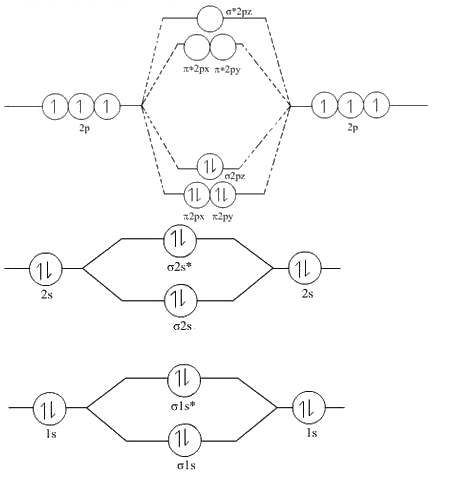
Step 2:
So According to the above molecular orbital diagram
The order of energies of molecular orbitals of N2 is as follows:

© 2026 GoodEd Technologies Pvt. Ltd.