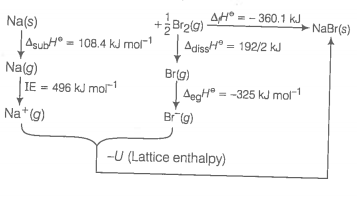
Use the following data to calculate for NaBr. for sodium metal, ionisation enthalpy of sodium, electron gain enthalpy of bromine, bond dissociation enthalpy of bromine, for NaBr(s)

© 2026 GoodEd Technologies Pvt. Ltd.