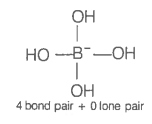
The geometry of a complex species can be understood from the knowledge of the type of hybridization of orbitals of the central atom. The hybridization of orbitals of a central atom in and the geometry of the complex are respectively
| 1. | sp3, tetrahedral | 2. | sp3, square planar |
| 3. | sp3d2, octahedral | 4. | dsp2, square planar |

© 2026 GoodEd Technologies Pvt. Ltd.