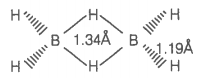
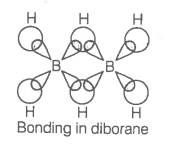
Which of the following statements are correct?
(Answer on the basis of the given figure.)

| a. | The two bridged hydrogen atoms and the two boron atoms lie in one plane |
| b. | Out of six B-H bonds, two bonds can be described in terms of 3-center-2-electron bonds |
| c. | Out of six B-H bonds, four B-H bonds can be described in terms of 3 center 2 electron bonds |
| d. | The four-terminal B-H bonds are two center-two electron angular bonds |
Choose the correct option
1. (a, b, c)
2. (b, c, d)
3. (a, c, d)
4. (a, b, d)

© 2026 GoodEd Technologies Pvt. Ltd.