The electrode potential for the Mg electrode varies according to the equation:
\(E_{Mg^{2+}/Mg}\ = \ E_{Mg^{2+}/Mg}^{o} \ - \ \frac{0.059}{2}log\frac{1}{[Mg^{2+}]}\)
The graph of EMg2+ / Mg vs log [Mg2+] among the following is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
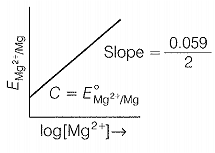

© 2026 GoodEd Technologies Pvt. Ltd.