Match the graph given in Column I with the order of reaction given in Column II.
More than one item in Column I may be linked to the same item in Column II:
| Column I | Column II | ||
| (i) |  |
(a) | 1st order |
| (ii) |  |
(b) | Zero order |
| (iii) |  |
||
| (iv) |  |
||
| (i) | (ii) | (iii) | (iv) | |
| 1. | (a) | (b) | (a) | (b) |
| 2. | (a) | (b) | (b) | (a) |
| 3. | (a) | (a) | (b) | (b) |
| 4. | (b) | (b) | (a) | (a) |
A = -kt + [R]0 ...(i)
Which denotes a straight line equation similar to y = mx + C
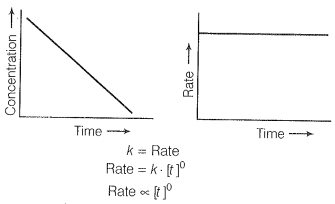
For a first-order reaction [concentration]
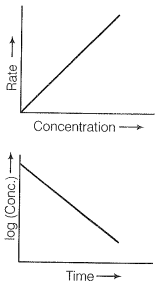
Therefore the graph between rate and concentration may be drawn as

© 2026 GoodEd Technologies Pvt. Ltd.