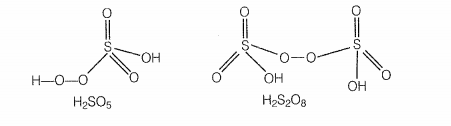
Which of the following are peroxoacids of sulphur?
| 1. | \(\mathrm{H_{2} SO_{5} } \text{ and } \mathrm{H_{2} S_{2} O_{8}}\) | 2. | \(\mathrm{H_{2} SO_{5 }} \text{ and } \mathrm{H_{2} S_{2} O_{7}}\) |
| 3. | \(\mathrm{H_{2} S_{2} O_{7}} \text{ and } \mathrm{H_{2} S_{2} O_{8}}\) | 4. | \(\mathrm{H_{2} S_{2} O_{6}} \text{ and } \mathrm{H_{2} S_{2} O_{7}}\) |
Hint: Peroxoacid contains -O-O- linkage.


© 2026 GoodEd Technologies Pvt. Ltd.