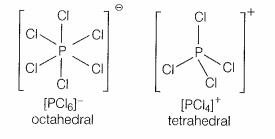
In solid-state, PCl5 exists as:
| 1. | Covalent solid. |
| 2. | Octahedral structure. |
| 3. | Ionic solid with [PCl6]+ octahedral and [PCl4]- tetrahedral. |
| 4. | Ionic solid with [PCl4]+ tetrahedral and [PCl6]- octahedral. |

© 2026 GoodEd Technologies Pvt. Ltd.