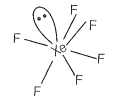
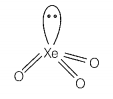
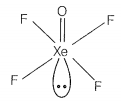
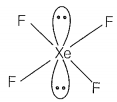
Match the compounds given in Column I with the hybridisation and shape given in Column II and mark the correct option.
| Column I | Column II | ||
| A | XeF6 | 1. | sp3d3 distorted octahedral |
| B. | XeO3 | 2. | sp3d2 square planar |
| C. | XeOF4 | 3. | sp3 pyramidal |
| D. | XeF4 | 4. | sp3d2 square pyramidal |
Codes:
| Options: | A | B | C | D |
| 1. | 1 | 3 | 4 | 2 |
| 2. | 1 | 2 | 4 | 3 |
| 3. | 4 | 3 | 1 | 2 |
| 4. | 4 | 1 | 2 | 3 |
|
S.No |
Compound |
Hybridisation |
|
A. B. C. D. |
|
sp3d3 -distorted octahedral sp3 pyramidal sp3d2 -square pyramidal sp3d2 -square planar |

© 2026 GoodEd Technologies Pvt. Ltd.