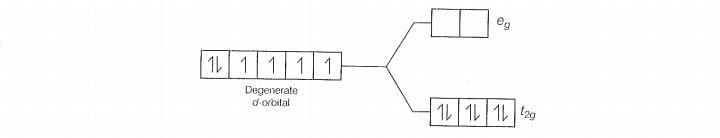
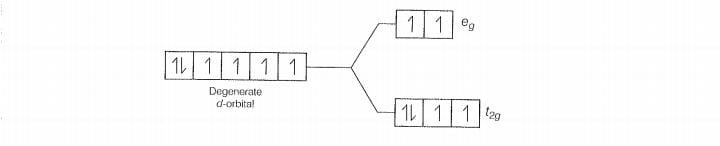
Q. 30 Give the electronic configuration of the following complexes on the basis
of crystal field splitting theory.
increasing field strength i.e.,F- < NH, <CN
Hence. CN and NH, being strong field ligand pair up t, electrons before filing e set

© 2026 GoodEd Technologies Pvt. Ltd.