Explain why
has a value of only 1.74 BM?
Ans/.
involves hybridisation with one unpaired electron (as shown by its
magnetic moment 1.74 BM) and involves sphybridisation with five
unpaired electrons (because magnetic moment equal to 6.92 BM)
CN is stronger ligand than H,O according to specttochemical series. for CNhence,
fourth electron will pair itself, Whereas for water pairing will not happen for the
electronic configuration of Fe is
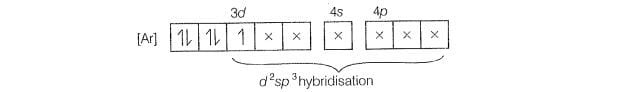
One unpaired electron
For
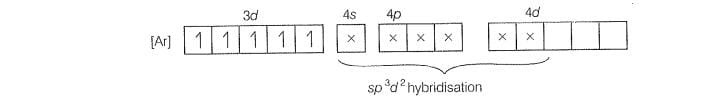
Five unpaired electron
Hence, and are inner obital an douter orbital complex respectively

© 2026 GoodEd Technologies Pvt. Ltd.