Q. 73 Aryl halides are extremely less reactive towards nucleophilic substitution.
Predict and explain the order of reactivity of the following compounds
towards nucleophilic substitution.
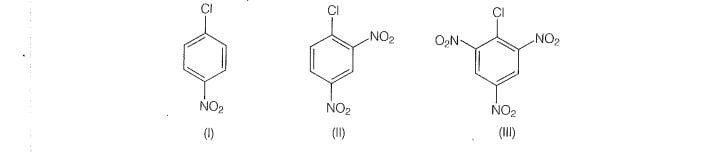
electron withdrawing group at ortho and para position increases the stability of
intermediates and hence increases the reactivity of aryl halides towards nucleophilic
substitution reaction.
Now, more the number of EWG al ortho and para position, higher will be the reactivity of aryl
halide. Compound (lll) has three EWG so, it is most reactive and compound (|) has only one
EWG so. its least reactive, So, the order of reactivity is (i) <(l) <(iii)

© 2026 GoodEd Technologies Pvt. Ltd.