Which of the following species can act as the strongest base?
| 1. | \(^\ominus \mathrm{OH}\) | 2. | \(^\ominus \mathrm{O R}\) |
| 3. | \({ }^{\ominus} \mathrm{OC}_6 \mathrm{H}_5\) | 4. |  |
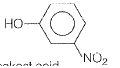 is the acid of
is the acid of 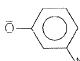 Among all these acids, ROH is the weakest acid.
Among all these acids, ROH is the weakest acid.
Therefore, the strongest base is and the correct option is (2)

© 2026 GoodEd Technologies Pvt. Ltd.