Select the correct option based on statements below:
| Assertion (A): | Ethanol is a weaker acid than phenol. |
| Reason (R): | Sodium ethoxide may be prepared by the reaction of ethanol with aqueous NaOH. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
The conjugate base of phenol, that is, phenoxide ion is stabilized by resonance but ethoxide ion is destabilized by the +I effect of the alkyl chain. Hence, ethanol is a weaker acid than phenol.
Resonance in phenoxide ion as follows:
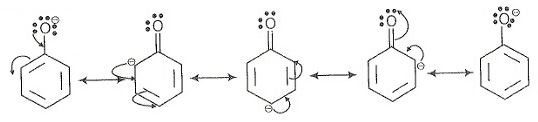
Sodium ethoxide cannot be prepared by the reaction of ethanol with aqueous NaOH because ethanol is a weak acid and for ethoxide ion formation strong base is required.

© 2026 GoodEd Technologies Pvt. Ltd.