13.9 Give the structures of A, B and C in the following reactions:
(i) 
(ii) 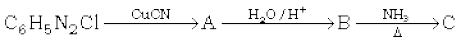
(iii) 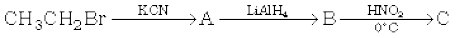
(iv) 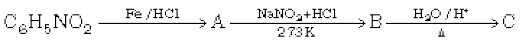
(v) 
(vi) 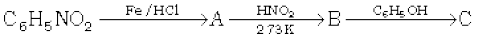
(i)
In the first reaction, ethyl iodide reacts with NaCN, and a substitution reaction occurs. The product obtained is propanenitrile. In the next step, partial hydrolysis of propanenitrile takes place and an amide is obtained as a product.
In the last step, the Hoffmann bromamide reaction takes place and methanamine is obtained as a product.
The reactions are as follows:
(ii)
In the first reaction, benzene diazonium chloride reacts with CuCN, and benzonitrile is obtained as a product. In the second reaction, hydrolysis of the CN group takes place in an acidic medium and benzoic acid is obtained as a product.
The benzoic acid reacts with ammonia followed by strong heating and a benzamide is obtained as a product. The reactions are as follows:
(iii)
(iv)
In the first reaction, nitrobenzene undergoes a reduction reaction when reacts with Fe/HCl, then Aniline is obtained as a product. Aniline undergoes a diazotization reaction and benzenediazonium chloride is formed as a product.
In the last step, benzenediazonium salt reacts with water, and phenol is obtained as a product.
The reaction is as follows:
(v)
In the first reaction, ammonia reacts with ethanoic acid and gives ammonium salt which on further heating at high temperature gives amides.
In the next step, amide reacts with NaOBr, and methanamine is obtained as a product. In the last step, methanamine, reacts with NaNO2, HCl, and forms unstable diazonium salt.
Then diazonium salt further reacts with water and form methanol as a product.
The reactions are as follows:
(vi)
In the step, reduction of NO2 group takes place, when nitrobenzene reacts with Fe, HCl and aniline are obtained as a product. In the next step, aniline reacts with HNO2 at 273 K , and benzenediazonium chloride is obtained as a product.
In the last step, benzenediazonium chloride reacts with phenol and azo dye is obtained as a product.
The reactions are as follows:

© 2026 GoodEd Technologies Pvt. Ltd.





