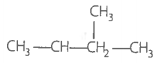
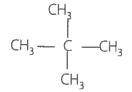
Match the hydrocarbons in Column I with the boiling points given in Column II.
| A. n-Pentane | 1. 282.5 K |
| B. Isopentane | 2. 309 K |
| C. Neopentane | 3. 301 K |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 2 | 1 | 3 |
| 4. | 3 | 2 | 1 |
|
Column I |
Column II |
|
A. n-pentane |
309 K due to no branch |
|
B. iso-pentane |
301 K due to one branch
|
|
C. neo-pentane |
282.5 K due to two branches
|

© 2026 GoodEd Technologies Pvt. Ltd.