Which of the following species absorb maximum energy in its HOMO-LUMO electronic transition?
In which of the following transformations, the bond order has increased and the magnetic behavior has changed?
The correct order of Cl-O bond order is
The shape of is :
1. Trigonal planar
2. Pyramidal
3. Bent T-shape
4. See-saw
The correct statement regarding molecule is:
1. two bonds
2. molecules has 2 lone pair, 2 bonds and 2 bonds
3. two bonds
4. one and one bond
The correct order of increasing bond angles in t
he following species is
1.
2.
3.
4.
The correct shape and hybridization for XeF4 are:
1. octahedral, sp3d2
2. trigonal bipyramidal, sp3d3
3. planar triangle, sp3d3
4. square planar, sp3d2
A diatomic molecule, among the following species, that only has bonds according to the Molecular Orbital Theory (MOT) is:
| 1. | Be2 | 2. | O2 |
| 3. | N2 | 4. | C2 |
CaO and NaCl have the same crystal structure and approximately the same ionic radii. If U is the lattice energy of NaCl, the approximate lattice energy of CaO is :-
1. U/2
2. U
3. 2 U
4. 4 U
In bisulphate ion, HSO4-, the formal charge on S atom is
1. +6
2. +4
3. +1
4. +2
Which has non-zero dipole moment?
1. PCl5
2. PCl3F2
3. PCl3Br2
4. PCl3(CH3)2
Which is incorrect about bond angles?
1. NH3 > NF3
2. NF3 < PF3
3. NH3 > PH3
4. NH3 > H2O
The correct statement is
1. When O2 is converted into then bond distance increases
2. When N2 is converted into then bond distance increases
3. When CO is converted into CO+ then bond distance increases
4. All of these
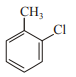
In which case observed dipole moment is greater than theoretical dipole moment?
1. 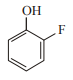
2. 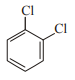
3. 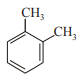
4.